Our products
Scalable Freeze-Drying Solutions from R&D to GMP
RheaVita offers a range of freeze-dryer models covering R&D as well as GMP.
The RheaLyo™ PAT approach reduces tech transfer efforts significantly. With a high level of transferability, moving your drug product or vaccine through the lyophilization scaling process will save you time and money.
Get in touch for customized solutions or order a pre-engineering study for exploring the implementation of a RheaLyo™ GMP-Flex line in your facility.
Product range features
- Product name
- GMP?
- # Spin-freeze
positions - # Drying positions
- Estimated daily capacity
range (24h)
- RheaLyo™ Mono Freeze-Dryer
- No
- 1
- 1
- ≈1-6
- RheaLyo™ Multi Freeze-Dryer
- No
- 2
- 12
- ≈50-100
- RheaLyo™ GMP-Flex “4/120”
- Yes
- 4
- 120
- ≈620-1,610
- RheaLyo™ GMP-Flex “16/240”
- Yes
- 16
- 240
- ≈1,250-5,640
- RheaLyo™ GMP-Flex “24/600”
- Yes
- 24
- 600
- ≈3,130-9,670
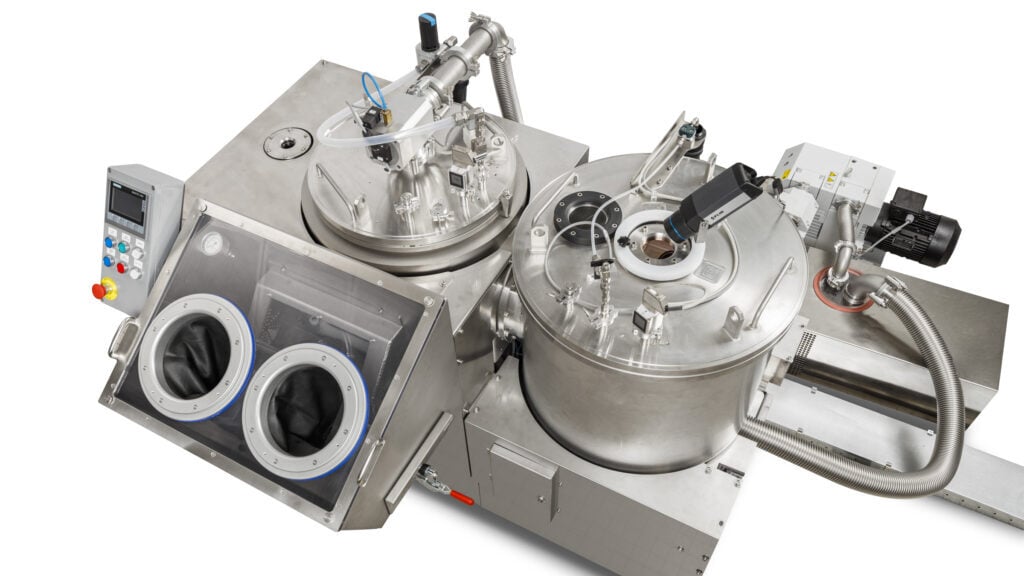
RheaLyo™ Mono freeze-dryer
The RheaLyo™ Mono system accelerates R&D and formulation development through rapid, small-scale continuous freeze-drying.
Optimized processes are easily scaled for GMP production, significantly reducing tech transfer and time-to-market.

RheaLyo™ Multi freeze-dryer
The RheaLyo™ Multi system streamlines pre-clinical production with minimal tech transfer and direct scalability to GMP lines.
Advanced controls ensure pre-clinical quality closely matches future GMP runs.
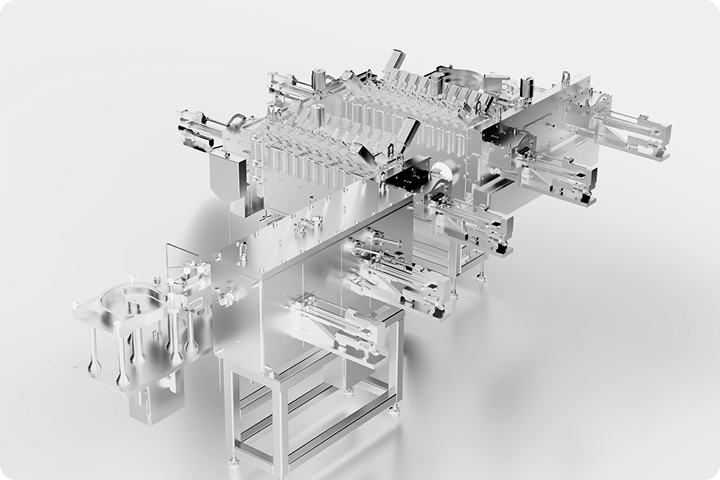
GMP-Flex continuous freeze-dryers
RheaLyo™ GMP-Flex is the only commercial continuous freeze-drying system for GMP pharmaceutical production.
Robotic automation ensures precise, high-capacity vial processing under controlled conditions.


Schedule a consultation with our experts to explore the best solutions for your needs.
Discover our services
RheaVita offers comprehensive continuous freeze‑drying services—from feasibility studies and formulation development to process optimization.

