
Feasibility studies
Feasibility Studies for Continuous Freeze-Drying of Your Formulation
RheaVita has the necessary expertise in controlled, continuous freeze-drying to conduct feasibility studies with your formulation of interest.
Without the need to invest in equipment you can explore the impact of the RheaLyo™ freeze-drying methodology on your specific drug product or vaccine.
The objective of such a study is to evaluate the feasibility to continuously freeze-dry your formulation.

Optimize Your Freeze-Drying Process with RheaLyo™ Controlled Technology
The RheaLyo™ controlled and continuous freeze-drying technology is inherently conducted applying optimal settings based on the integrated control loop during primary drying, allowing fast sublimation without exceeding the critical product temperature.
With the possibility to vary the duration of the crystallization phase, the optimal freezing settings for your product can be determined.
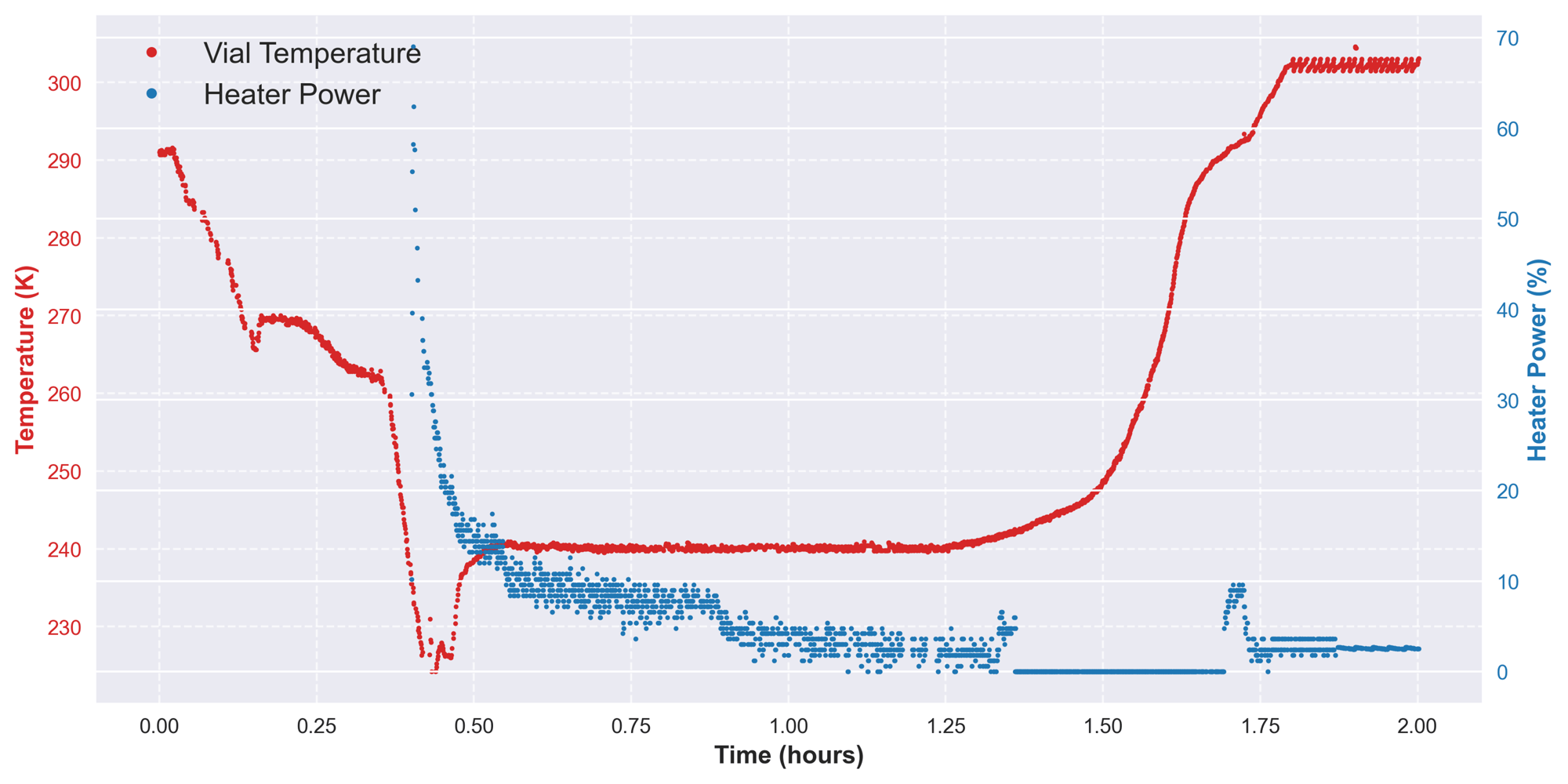
Feasibility Studies with PAT Monitoring and Rapid Sample Delivery
Throughout the feasibility study, PAT tools for process monitoring and control will be applied as indicated in an experimental plan.
Contact us for a quote and learn how quickly we can provide you with dried samples for your analytical work.

Discover our products
Latests news
Stay in the know.




-1.png)

-2.png)




